| | Enthalpy from Bond Energy | |
To determine the enthalpy of a reaction using bond energy DHo(reaction) =SH(bonds broken) - SH(bonds formed) Break the reactants and make the products. Each bond must be accounted for. 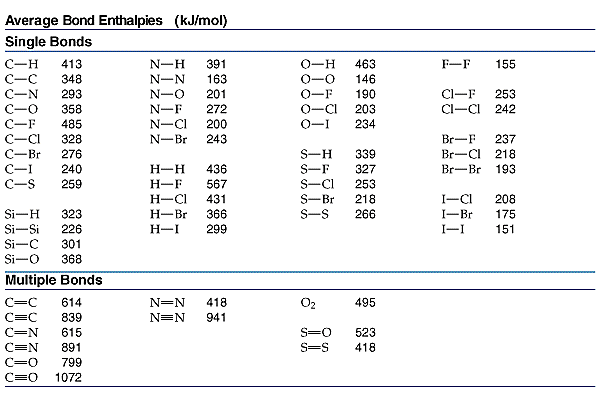
Calculate the  H for the following reaction in the gas phase. H for the following reaction in the gas phase. 
DH | =SH(bonds broken) - SH(bonds formed) | DH | =(2(N-H) + (N=N) + 2(O-H)) - (2(N-H) + (N-O) + (O-H) + 3(N-H)) | DH | =(2(391)+418)+2(463)) - (2(391) + 201 + 463 + 3(391)) | DH | =2126-2619= -493kJ |
 Chemical Demonstration Videos Chemical Demonstration Videos
| 
